How and why humans exist are questions that have challenged different cultures ever since the dawn of civilization. Many scientists have taken upon their shoulders the duty of answering these fundamental questions, coming up with several especially creative ideas over the past few decades.
One prominent idea is that life originated in or near hydrothermal vents, which are cracks in the seafloor that continuously spit out heated water into the ocean from underground. These cracks are located deep inside volcanically active regions in the seafloor where the Earth’s tectonic plates, massive slabs of rock that make up the Earth’s outer shell, are moving apart from each other. In these regions, magma comes very close to the seafloor and heats sea water, sometimes well above boiling. This heated water carries molecules that have been transformed via interactions with the surrounding seafloor environment, possibly helping to create simple life. Assuming this idea is correct, the critical question would be: how would this have happened?
In a recent study, scientists from the Tokyo Institute of Technology and the Blue Marble Space Institute of Science researched how pyrite (“fools gold”), a common mineral composed of iron and sulfur, could have influenced the attachment and detachment of the building blocks of life. Pyrite is a mineral that is produced when sulfur, which is soluble in the hot water emanating from hydrothermal vents, attaches to iron suspended in the surrounding cold ocean water. This newly produced mineral then accumulates at the bottom of the ocean, creating abundant pyrite mineral surfaces. The project was an extension of a previous study whose purpose was to probe the binding strengths of some simple building blocks of life, such as amino acids.

This is pyrite. Source: Wikimedia Commons. CarlesMillan / CC BY-SA
The researchers studied how the simple building blocks of life may have attached to these mineral surfaces and engaged in further reactions. These reactions can produce more complex molecules that may have been important for the emergence of life. They used a method called atomic force microscopy, which uses a laser-pointed needle dragged along a sample to determine what the surface of any material looks like. Although it is commonly used to image and understand molecules, the researchers used this method to measure the forces between molecules and mineral surfaces which determine how strongly they attach.
Using their measurements and computer simulations, they found that the main forces determining the strength of attachment of amino acids to pyrite were because of magnet-like properties of the molecules interacting with each other. Just like the poles of magnets, molecules with opposite charges attract each other and molecules with identical charges repel each other. Pyrite and the building blocks of life seem to behave like tiny magnets in these oceanic scenarios, in that they may be attracted or repelled from each other.
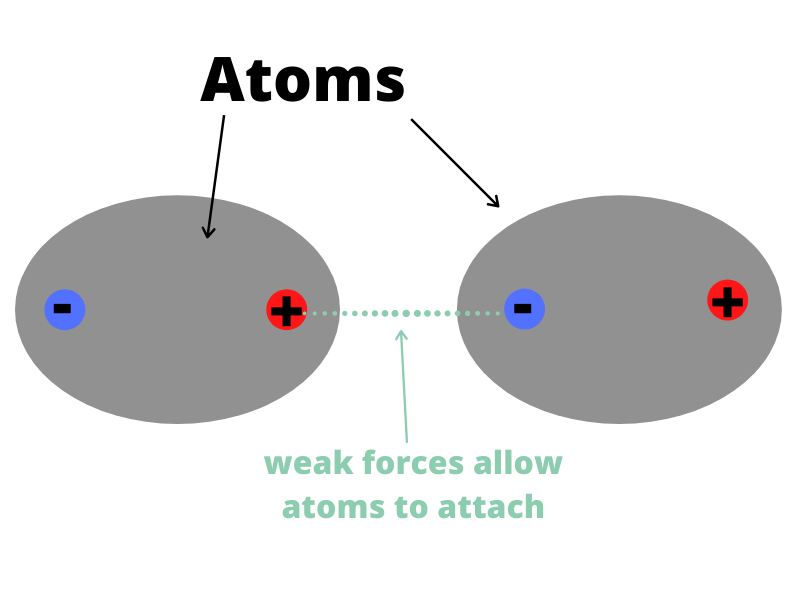
This is a diagram of how two atoms can be attracted to one another. Electrons are always swirling around atoms. When they cluster to one side of an atom, that side becomes negative. They attract the side of another atom where electrons are less clustered and more positive. Image source: Sciworthy
The researchers also found that these force values are similar to the forces found in the structures inside our cells. These structures make important mechanisms for life possible, such as protein production, DNA repair, and the communication between cells. In the future, these researchers plan to extend this work by recreating this pyrite-amino acid attachment with different factors and conditions.
Understanding how the building blocks of life can attach and detach from pyrite gives us insight on how these simple molecules could create complex systems that would eventually originate life. With a better understanding of these mechanisms, perhaps someday the pieces will fit and we will gain the answers to our questions.


