Some 56 million years ago, a massive pulse of carbon dioxide into the atmosphere sent global temperatures soaring. In the oceans, carbonate sediments dissolved, some organisms went extinct and others evolved.
Scientists have long suspected that ocean acidification caused the crisis—similar to today, as manmade CO2 combines with seawater to change its chemistry. Now, for the first time, scientists have quantified the extent of surface acidification from those ancient days, and the news is not good: the oceans are on track to acidify at least as much as they did then, only at a much faster rate.
In a study published in the latest issue of Paleoceanography, the scientists estimate that ocean acidity increased by about 100 percent in a few thousand years or more, and stayed that way for the next 70,000 years. In this radically changed environment, some creatures died out while others adapted and evolved. The study is the first to use the chemical composition of fossils to reconstruct surface ocean acidity at the Paleocene-Eocene Thermal Maximum (PETM), a period of intense warming on land and throughout the oceans due to high CO2.
“This could be the closest geological analog to modern ocean acidification,” said study coauthor Bärbel Hönisch, a paleoceanographer at Columbia University’s Lamont-Doherty Earth Observatory. “As massive as it was, it still happened about 10 times more slowly than what we are doing today.”
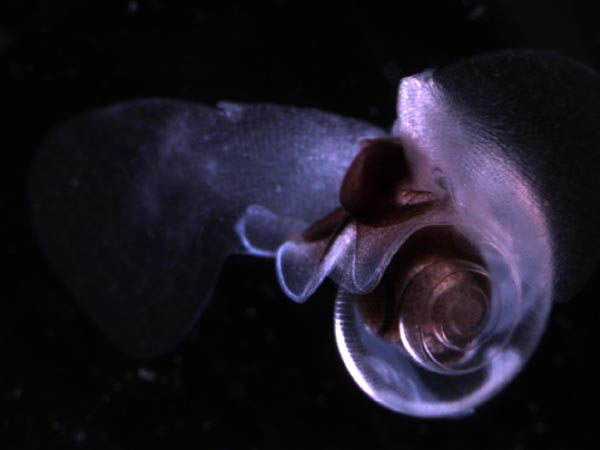
The oceans have absorbed about a third of the carbon humans have pumped into the air since industrialization, helping to keep earth’s thermostat lower than it would be otherwise. But that uptake of carbon has come at a price. Chemical reactions caused by that excess CO2 have made seawater grow more acidic, depleting it of the carbonate ions that corals, mollusks and calcifying plankton need to build their shells and skeletons. Ocean acidification in the modern ocean is already affecting some marine life, as shown by the partly dissolved shell of this planktic snail, or pteropod, caught off the Pacific Northwest. (Nina Bednaršek/NOAA)
In the last 150 years or so, the pH of the oceans has dropped substantially, from 8.2 to 8.1–equivalent to a 25 percent increase in acidity. By the end of the century, ocean pH is projected to fall another 0.3 pH units, to 7.8. While the researchers found a comparable pH drop during the PETM–0.3 units–the shift happened over a few thousand years.
“We are dumping carbon in the atmosphere and ocean at a much higher rate today—within centuries,” said study coauthor Richard Zeebe, a paleoceanographer at the University of Hawaii. “If we continue on the emissions path we are on right now, acidification of the surface ocean will be way more dramatic than during the PETM.”
The study confirms that the acidified conditions lasted for 70,000 years or more, consistent with previous model-based estimates. “It didn’t bounce back right away,” said Timothy Bralower, a researcher at Penn State who was not involved in the study. “It took tens of thousands of years to recover.”
From seafloor sediments drilled off Japan, the researchers analyzed the shells of plankton that lived at the surface of the ocean during the PETM. Two different methods for measuring ocean chemistry at the time—the ratio of boron isotopes in their shells, and the amount of boron –arrived at similar estimates of acidification. “It’s really showing us clear evidence of a change in pH for the first time,” said Bralower.
What caused the burst of carbon at the PETM is still unclear. One popular explanation is that an overall warming trend may have sent a pulse of methane from the seafloor into the air, setting off events that released more earth-warming gases into the air and oceans. Up to half of the tiny animals that live in mud on the seafloor—benthic foraminifera—died out during the PETM, possibly along with life further up the food chain.
Other species thrived in this changed environment and new ones evolved. In the oceans, dinoflagellates extended their range from the tropics to the Arctic, while on land, hoofed animals and primates appeared for the first time. Eventually, the oceans and atmosphere recovered as elements from eroded rocks washed into the sea and neutralized the acid.
Today, signs are already emerging that some marine life may be in trouble. In a recent study led by Nina Bednaršek at the U.S. National Oceanic and Atmospheric Administration, more than half of the tiny planktic snails, or pteropods, that she and her team studied off the coast of Washington, Oregon and California showed badly dissolved shells. Ocean acidification has been linked to the widespread death of baby oysters off Washington and Oregon since 2005, and may also pose a threat to coral reefs, which are under additional pressure from pollution and warming ocean temperatures.
“Seawater carbonate chemistry is complex but the mechanism underlying ocean acidification is very simple,” said study lead author Donald Penman, a graduate student at University of California at Santa Cruz. “We can make accurate predictions about how carbonate chemistry will respond to increasing carbon dioxide levels. The real unknown is how individual organisms will respond and how that cascades through ecosystems.” Other authors of the study, which was funded by the U.S. National Science Foundation: Ellen Thomas, Yale University; and James Zachos, UC Santa Cruz.
Are you the author of this article? We had a site crash back in 2016 and lost some author attributions. We promise this is not a snub! Please email us and let us know that this is your post. Thanks and apologies!