Alzheimer’s disease is a form of dementia in which memory and cognitive skills decline over time as brain cells progressively die off throughout the course of the disease. It is the most common cause of dementia, affecting one out of every nine people aged 65 and older. It is the sixth leading cause of death in the United States. Despite the prevalence of Alzheimer’s disease and the burden it causes on patients, caregivers, and the healthcare system, the best treatment for the disease is symptom management.
Commonly prescribed Alzheimer’s disease drugs such as galantamine, rivastigmine, and donepezil are able to only reduce or control some of the cognitive and behavioral symptoms of the disease for a time, but there are no widely used medications that can actually modify or delay disease progression. Indeed, the attempts to bring a new Alzheimer’s disease treatment to patients have been met with many failures as drugs fail to demonstrate efficacy in Phase III clinical trials. In fact, only one new medication has been granted conditional approval by the FDA in the last two decades, and the efficacy and impact of that drug is still under investigation by the scientific community.
Nonetheless, researchers remain determined to bring disease-modifying treatments to Alzheimer’s disease patients. A number of trials are focused on one of the pathological hallmarks of Alzheimer’s disease, a protein called tau, which is known to accumulate and tangle in the brains of those with Alzheimer’s disease. At the University of Atlanta, a recent completion of a Phase II clinical trial evaluated whether the repurposing of an ADHD medication called atomoxetine may have a benefit for Alzheimer’s disease treatment.
The researchers’ rationale was that Alzheimer’s disease typically starts in a part of the brain called the locus coeruleus, which is located in the brainstem. Disease-linked forms of the tau protein appear in the locus coeruleus before any other parts of the brain, and the rate of locus coeruleus decline predicts the amount of pathological tau which accumulates. Atomoxetine increases the amount of a brain chemical called norepinephrine, which is produced in the locus coeruleus. Thus, the supplementation of norepinephrine which is lost over Alzheimer’s disease progression may prove beneficial in the treatment of the disease.
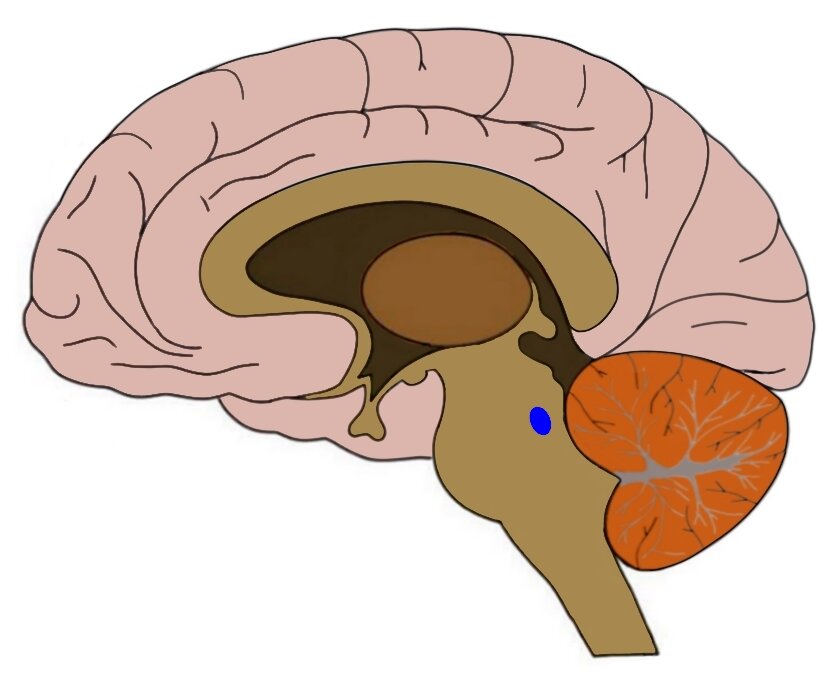
The blue dot is where the locus coeruleus is located. Source
In this proof-of-concept study, 39 participants with early Alzheimer’s disease were recruited and examined over one year. Participants were between the ages of 50 and 90, and were randomly assigned to either receive atomoxetine or a placebo. After 6 months, those that were receiving atomoxetine before were switched to the placebo group, and vice versa. The researchers collected samples from the participant’s cerebrospinal fluid and plasma at the start of the study, again at 6 months, and again at 12 months. The participants also received brain scans.
Crucially, a significant reduction in the amount of tau protein present in the participants receiving atomoxetine was observed. In addition, the researchers examined a broad range of biological markers associated with different types of brain health, such as brain immune function and inflammation. They found that atomoxetine treatment appeared to improve those measures as well. Intriguingly, through fMRI measurement, brain connectivity between select regions also appeared to be improved as a result of atomoxetine treatment.
The primary aims of this study were to assess the safety of atomoxetine treatment in Alzheimer’s disease patients. The researchers explained that the small sample size and short time frame of the study would make it unlikely to detect disease-modifying outcomes such as improved cognitive ability. Measuring these outcomes requires a different type of study.
The study authors suggest that these initial results make atomoxetine an interesting candidate for further study for Alzheimer’s disease treatment in larger and longer term trials. The added value of repurposing already FDA-approved drugs for Alzheimer’s disease means that if proven to be effective for the disease, these medications may be able to be of benefit to patients faster, streamlining the lengthy drug development process. As Alzheimer’s disease patients wait for new treatments to become available, the study of promising candidates continues.