Coronavirus Disease 2019 (COVID-19) is a viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus prefers to replicate in the human upper respiratory tract where the temperature is optimal. Since SARS-CoV-2 affects so many body systems, researchers were interested in the types of tissues that the virus likes best. In this study, they specifically experimented with immune cells located inside the lower respiratory tract called myeloid cells.
The importance of studying myeloid cells relies on their key role in both innate and adaptive immunity. They originally come from our bone marrow, and are widely distributed in all tissues by way of the blood, protecting us from infection. If a virus infiltrates and replicates inside these cells, it will fool the immune system and prevent it from reacting in a timely manner. The virus will be easily transmitted to other tissues, promoting more cell destruction.
Whereas the presence of SARS-CoV-2 inside these myeloid cells had not been studied before, a previous experiment was done in 2006 studying a virus that is genetically similar to SARS-CoV-2 called SARS-CoV-1. Researchers found SARS-CoV-1 virus particles inside a human myeloid cell called monocyte, which eventually converts into a cell type that acts as the body’s first line of defense: macrophages. Macrophages are white blood cells that engulf whatever the body does not recognize as safe. Although this finding gives us a clue about what to expect of SARS-CoV-2, it is not enough evidence for us to know whether SARS-CoV-2 is also found in myeloid cells of infected people. We need to see the virus itself.
To find the virus at the scene, scientists at University of Pittsburgh Medical Center studied 7 patients who were on ventilators due to severe COVID-19 pneumonia. These were very sick patients. In fact, two of them passed away by the end of the experiment and three required a machine that pumps and oxygenates a patient’s blood outside the body.
As mentioned before, their goal was to collect myeloid cells from their lower respiratory tract and see if SARS-CoV-2 had infiltrated inside them. To collect cells, technicians quickly ejected sterile saline solution into the patient’s lungs and then immediately suctioned it back out. They preserved the liquid with paraformaldehyde and isolated the myeloid cells from the rest of the sample. After preparing them for testing, they looked at the cells using three kinds of microscopes: electron microscopy, light microscopy, and immunofluorescence.
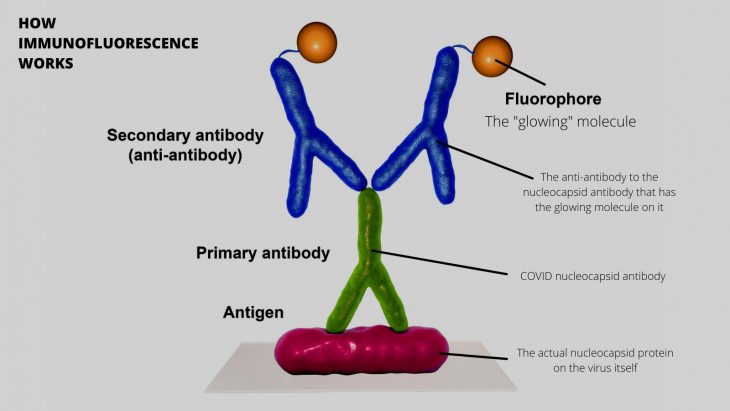
How immunofluorescence works, in general. Microscopy allows even more resolution, letting you see the cells where this is happening. This is how the scientists were able to see the virus without culturing or molecular detection! Diagram Source: Sciworthy
Immunofluorescence is a technique that uses certain antibodies to visualize the presence of particles like viruses by causing an immune response via immune molecules called antigens. Antigens bind to specific antibodies. With this technique, if a certain antigen is present in a sample, the fluorescent antibody will bind and release light. That will confirm the presence of the antigen. In this case they used antibodies against the SARS-CoV-2 nucleocapsid protein, immune molecules called CD14, CD16, CD142, and interleukin-6 (IL-6). The SARS-CoV-2 nucleocapsid protein is one of the major structural proteins of SARS-CoV-2 (different from the spike protein). The immune molecules mentioned are human proteins that are secreted by myeloid cells when there’s a foreign body such as a virus.
After detection, researchers captured and compared the cell images using a computer software, to measure how much of these molecules were expressed by the lung cells for each patient. The more immune molecules, the more inflammation. The results showed the presence of SARS-CoV-2 nucleocapsid protein inside myeloid cells called mononuclear and polymorphonuclear leukocytes. Researchers also found that myeloid cells with SARS-CoV-2 nucleocapsid protein were more likely to have the inflammatory markers that were studied (IL-6, CD14, and CD16).
The researchers confirmed that SARS-CoV-2 begins its viral infection in the upper airways and then infiltrates leukocytes found in the lower airways. This way, the virus fools the immune system and disperses to other tissues, aggravating the infection. As a defense mechanism, leukocytes will begin to express proinflammatory molecules and will cause a destructive response. These discoveries can lead to new medical strategies and innovations for COVID-19 severe pneumonia treatment. Further research is needed to determine why SARS-CoV-2 survives in lung myeloid cells.
Do you like podcasts? We have one! It’s called Science Decoded. We did an episode about this study, and interviewed the lead author. Check it out here! Want to find us on your favorite streaming service? There are lots of ways to listen.